Molecular ‘fossils’ offer microscopic clues to the origins of life – but they take care to interpret
- Written by Caroline Lynn Kamerlin, Professor of Chemistry and Biochemistry, Georgia Institute of Technology
 ATP synthase is an enzyme that has been using phosphate to generate life’s energy for millions of years.Nanoclustering/Science Photo Library via Getty Images
ATP synthase is an enzyme that has been using phosphate to generate life’s energy for millions of years.Nanoclustering/Science Photo Library via Getty ImagesThe questions of how humankind came to be, and whether we are alone in the universe, have captured imaginations for millennia. But to answer these questions, scientists must first understand life itself and how it could have arisen.
In our work as evolutionary biochemists and protein historians, these core questions form the foundation of our research programs. To study life’s history billions of years ago, we often use clues called molecular “fossils” – ancient structures shared by all living organisms.
Recently, we discovered that an important molecular fossil found in an ancient protein family may not be what it seems. The dilemma centers, in part, on a simple question: What does it mean if a simple molecular structure – the fossil – is found in every single organism on Earth? Do molecular fossils point to the seeds that gave rise to modern biological complexity, or are they simply the stubborn pieces that have resisted erosion over time? The answers have far-reaching implications for how scientists understand the origins of biology.
Follow the phosphorus to follow life
Life is made of many different building blocks, one of the most important of which is the chemical element phosphorus. Phosphorus makes up part of your genetic material, powers complex metabolic reactions and acts as a molecular switch to control enzymes.
Phosphorus compounds – specifically a charged form called phosphate – have a number of unique chemical properties that other biological compounds cannot match. In the words of the pioneering organic chemist F.H. Westheimer, they are chemically able to “do almost everything.”
Their unique combination of stability, versatility and adaptability is why many researchers argue that following phosphorus is key to finding life. The presence of phosphorus both close to home – in the ocean or on one of Saturn’s moons – and in the farthest reaches of our galaxy is strong evidence for the potential for life beyond Earth.
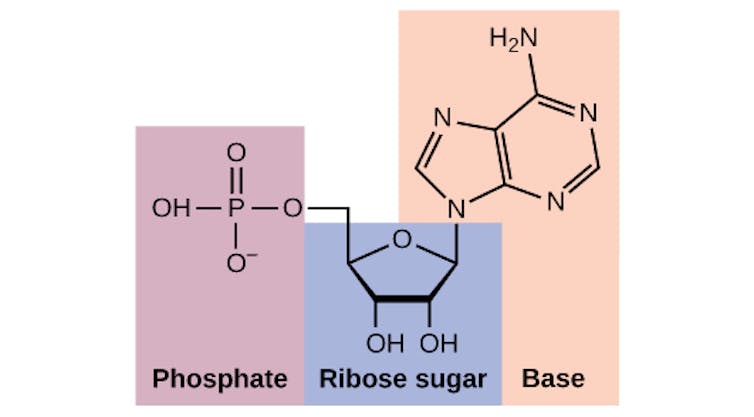 Phosphate is part of many essential biological molecules, including the building blocks of DNA.Charles Molnar and Jane Gair, CC BY-SA
Phosphate is part of many essential biological molecules, including the building blocks of DNA.Charles Molnar and Jane Gair, CC BY-SAIf phosphorus is so critical to life, how did early biology predating cells first use it?
Today, biological organisms are able to make use of phosphates through proteins – molecular machines that regulate all aspects of life. By binding to proteins, phosphates regulate metabolism and cellular communication, and they serve as a source of cellular energy.
Further, the process of phosphorylation, or adding a phosphate group to a protein, is ubiquitous in biology and allows proteins to perform functions their individual building blocks cannot. Without proteins, the existence of organisms such as bacteria and humans may not be possible.
Given how essential phosphorus is to life, scientists hypothesize that phosphate binding was among the first biological functions to emerge on Earth. In fact, current evidence suggests that the first phosphate-binding proteins are truly ancient – even older than the last universal common ancestor, the hypothetical mother cell to all life on Earth that existed around 4 billion years ago.
A mysterious phosphate-binding fossil
One family of phosphate-binding proteins, called P-loop NTPases, regulates everything from the communication between cells to the storage of energy and are found across the tree of life. Because P-loop NTPases are among the most ancient protein families, analyzing their properties can provide key insights into both the emergence of proteins and how primitive life used phosphates.
Although P-loop NTPases are diverse in structure, they share a common motif called a P-loop. This component binds to phosphate by wrapping a nest of amino acids – the building blocks that make up proteins – around the molecule. Every known organism has multiple families of P-loop NTPase, which makes the P-loop an excellent example of a molecular fossil that can provide clues about the evolution of life. Our crude analysis of the human genome estimates that humans have about 5,000 copies of P-loops.
When part of a larger protein structure, the P-loop folds like origami into a shape that is ideal for hugging a phosphate molecule. These nests are extremely similar to each other, even when the surrounding proteins are only distantly related in function. A landmark study in 2012 argued that even if the P-loop nest is extracted from a protein, it can still bind to phosphate. In other words, the ability of a P-loop to form a nest is determined by its interactions with phosphate, not its protein scaffold.
This study provided the first evidence that some forms of the P-loop sequence could have functioned billions of years ago, even before the emergence of large, complex proteins. If true, this implies that P-loop nests may have seeded the emergence and evolution of many of the phosphate-binding proteins seen today.
Interrogating the history of the P-loop
The pioneer of bioinformatics, Margaret Oakley Dayhoff, hypothesized in 1966 that the large collection of big proteins seen today arose from small peptides that were duplicated and fused over long periods of time. Although P-loops may have evolved in a different way, Dayhoff’s realization was the first to clarify how complex forms could have arisen from much simpler ones.
Inspired by Dayhoff’s hypothesis, we sought to interrogate the role that simple P-loops may have played in the evolution of the complex proteins key to life. Our findings challenge what’s currently known about these molecular fossils.
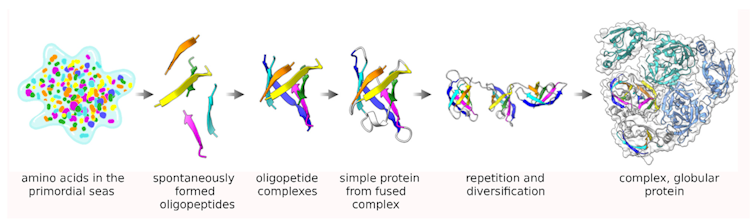 The Dayhoff hypothesis proposed that large, complex proteins arose from the duplication and merging of smaller, simpler peptides over time.Merski et al./Biomolecules, CC BY-SA
The Dayhoff hypothesis proposed that large, complex proteins arose from the duplication and merging of smaller, simpler peptides over time.Merski et al./Biomolecules, CC BY-SAUsing computer models, we compared a range of P-loops from the P-loop NTPase family to a control group made of the same amino acids but in a different order. While these control loops are also found in proteins, they do not form nests.
Although the P-loops and the control loops are very different in their nest-forming ability, we found that they both are able to form transient nests when embedded in proteins. This meant that, contrary to popular belief, the amino acid sequence of P-loops aren’t special in their ability to form nests – as would be expected if they alone were the seeds for many modern proteins.
A fossil eroded over time
Our work strongly suggests that while the P-loop is a molecular fossil, the true nature of its form billions of years ago may have been eroded by the sands of time.
For example, when we repeated our simulations in a different solvent – specifically methanol – we found that P-loops situated in their parent proteins were able to regain some of their ability to form nests. This doesn’t mean that being in methanol drove the first proteins with P-loops to form the nests critical for life. But it does emphasize the importance of considering the surrounding environment when studying peptides and proteins.
Just as archaeologists know to be careful in how they interpret physical fossils, historians of protein evolution could take similar care in their interpretation of molecular fossils. Our results complicate the current understanding of early protein evolution and, consequently, some aspects of the origins of life.
In resetting the field’s broader understanding of how these crucial proteins emerged, scientists are poised to start rewriting our own evolutionary history on this planet.
Caroline Lynn Kamerlin receives funding from the NASA Exobiology program.
Liam Longo receives funding from the NASA Exobiology program.
Authors: Caroline Lynn Kamerlin, Professor of Chemistry and Biochemistry, Georgia Institute of Technology